One Year’s Tax Revenue is Another Year’s Purchases of Drugs and Biologics
According to the Government Accountability Office’s (GAO) recently released “Snapshot of Government Spending,” the federal government spent about $637 billion on contracts for Fiscal Year 2021. Roughly 7.6% of all government contracting went to the purchases of drugs and biologics.1 Civilian agencies including the Department of Health and Human Services bought $13 Billion, and defense related agencies including the Department of Defense accounted for another $35.4 Billion. That’s a whopping $48.4 Billion to buy drugs and biologics in 2021. It also happens to be the exact amount the bottom 50 percent of US taxpayers (taxpayers with Adjusted Gross Income below $44,269) paid in individual federal income taxes in 2019.
Of course, some of that spending is attributable to the pandemic. (GAO found COVID-19 related spending increased from $35 Billion to $52 Billion in FY 2021.). But the pandemic doesn’t account for all of it. Adult Americans, many of whom are beneficiaries of government healthcare programs like Medicare, Medicaid, Tricare and the VA, take a lot of pills.
As of 2019, nearly 7 in 10 American adults aged 40–79 used at least 1 prescription drug in the past 30 days, and around 1 in 5 used at least 5 prescription drugs. The following chart illustrates the most commonly used drugs, by age group:
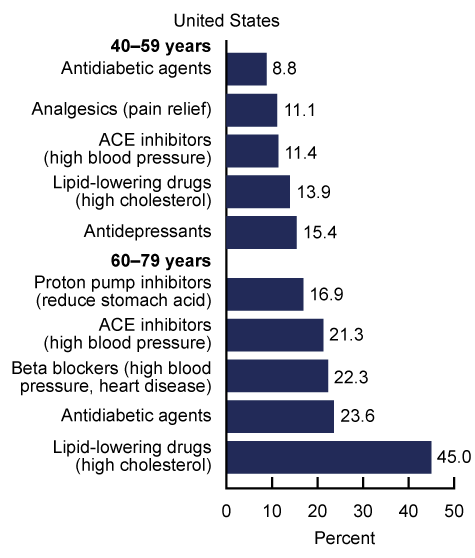
Many taxpayers would be happy to trace their tax dollars to paying for Grandma’s blood pressure pills, or Uncle Fred’s cholesterol meds. However, industry research shows that the government’s contracts to purchase drugs and biologics have long been, and continue to be, prone to fraud by pharmaceutical companies with billions in revenue every year thanks, in large part, to those taxpayer dollars.
In a groundbreaking industry case study published in 1984, before the modern amendments to the False Claims Act, John Braithwaite analyzed frauds including bribery (kickbacks), drug safety and unsafe manufacturing practices, antitrust violations, incentives to start patients on drugs, and financial abuses committed by pharmaceutical companies.2 Almost every company Braithwaite interviewed acknowledged dealing with illegal behavior.
Then Congress passed the False Claims Act in 1986. Thereafter, pharmaceutical companies improved practices and self-regulated to avoid FCA liability, right? Unfortunately, Braithwaite’s follow-up research in 2014 did not find fraud slowed down after his 1984 study let alone stop.3 Rather, his most significant finding was that the scale of pharmaceutical fraud increased as the prices paid by purchasers, including the US government, shot up.
Indeed, since 1986, settlements and judgments involving false claims in the sale of drugs and biologics by pharmaceutical companies to the United States have factored heavily in the government’s total recoveries, and the size of the settlements confirms Braithwaite’s findings of increased dollars at stake. Since 2000, every one of the top 10 pharmaceutical companies in 2021 (ranked by revenue) has inked more than one settlement to resolve “alleged” False Claims Act violations.
Together these Top 10 leaders in the pharma industry have returned more than $5.7 billion to the government over twenty years.4
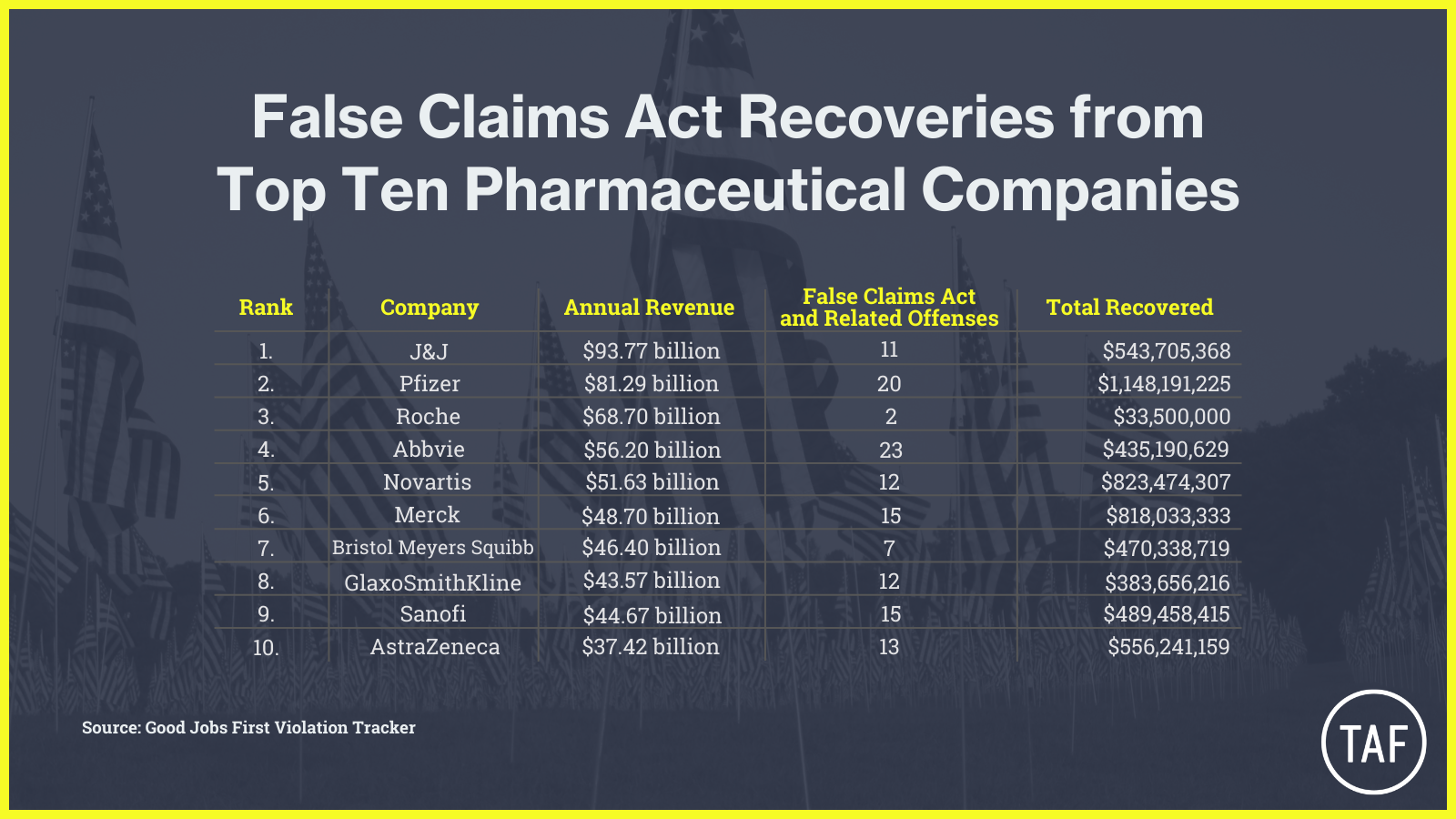
Note: Pharmaceutical companies often sell more than drugs and biologics. Many of them have robust divisions selling medical devices, which was covered in yesterday’s post. The offenses and recoveries summarized here may have involved devices, not just drugs or biologics.
Even with the False Claims Act and the massive recoveries made possible by whistleblowers exposing pharmaceutical fraud since 1986, the fraud weed has a deep root in 7.6% of all government contracts — the purchases of drugs and biologics.
Clearly, our weeding in this field isn’t done.
Written by Kate Scanlan of Keller Grover LLP. Tony Munter of Price Benowitz. Fact checked by Julia-Jeane Lighten of Taxpayers Against Fraud.
1. Drugs and biologics are regulated under the Food Drug and Cosmetic Act and must be approved as safe and effective by the FDA before they may be sold in interstate commerce. Biologics include “a wide range of products such as vaccines, blood and blood components, allergenics, somatic cells, gene therapy, tissues, and recombinant therapeutic proteins. … In contrast to most drugs that are chemically synthesized and their structure is known, most biologics are complex mixtures that are not easily identified or characterized.” https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers
2. Braithwaite J. Corporate crime in the pharmaceutical industry. London: Routledge & Kegan Paul pic; 1984.
3. Dukes G, Braithwaite J. Maloney JP. Pharmaceuticals, corporate crime and public health. Cheltenham: Edward Elgar Publishing; 2014.
4. Running afoul of the FCA is not something reserved for the industry’s Top 10, however. In just the last few months Biogen and Eli Lilly added more than a billion dollars to this total. In July, Biogen agreed to a $900 million settlement to resolve allegations regarding kickbacks in the sale of its drugs. Then, in August, a jury returned a $61.2 million verdict – before trebling – against Eli Lilly for failing to refund rebates to the Medicaid drug program.